A promising suite of precision radiotherapeutics.
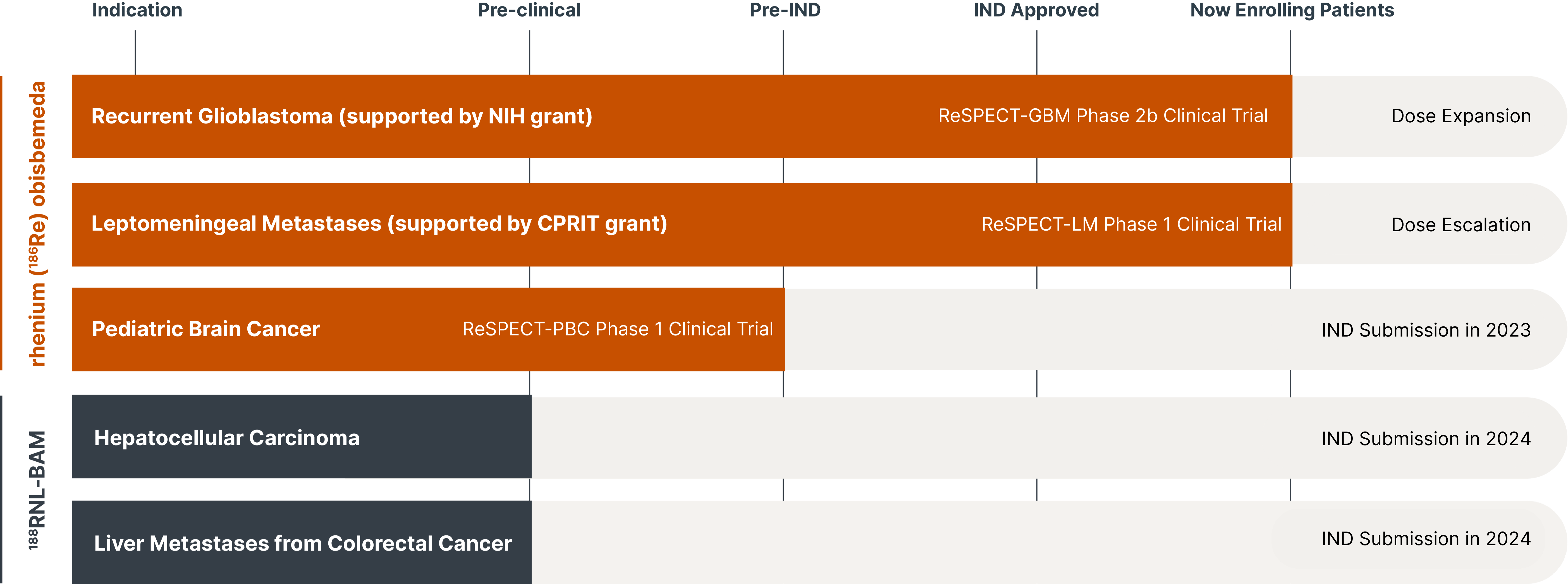
Leveraging novel drug formulations, we have developed a radiotherapeutic platform with the potential to lengthen and improve the lives of adults and children with challenging cancers. Taking a unique approach in combining radiation particles with nanoliposomes and alginate microspheres, we expect to target cancer more safely, precisely and effectively than other approaches in radiation therapy and oncology.